Proteostasis refers to the ability of cells to maintain protein homeostasis. Approaches to the contribution of proteostasis to disease have mostly assumed that regulation of proteostasis networks is a cell autonomous process. However, it is becoming apparent that organisms have evolved transcellular means of protection against proteotoxicity.
In our lab we are interested in exploring this new concept whereby tissues and organs cooperate to maintain proteostasis, using the retina as our privileged model. We are particularly interested in exosomes, in how these extracellular vesicles are critical vehicles in transferring proteotoxic material and/or machinery that supports proteostasis across cells and how, upon ageing, the ability to sustain a robust proteostasis network is lost.
To understand the compositions of exosomes and their function also means that we need to know the molecular mechanisms that regulate exosomes biogenesis. In our lab we are particularly interested in the mechanisms that mediated the triage of cytosolic proteins into nascent exosomes and how these mechanisms affect exosome-mediated intercellular communication.
During ageing and disease cells are exposed to increased levels of stress that damages various biomolecules, including cellular proteins. Dysregulation of proteostasis, including protein damage, often leads to the accumulation of toxic oligomers and insoluble protein aggregates intracellularly and/or in the extracellular space, frequently leading to disease.
In our lab we are interested in exploring a new concept whereby tissues and organs cooperate to maintain proteostasis, particularly in how cells can:
1) transfer proteostasis machinery, such as molecular chaperones and proteasome subunits, between them to help more vulnerable cells, thus improving tissue and organ fitness;
2) secrete proteotoxic material that can be uptaken and subsequently eliminated by neighboring cells. We are particularly interested in exosomes, in how these extracellular vesicles are critical vehicles in transferring proteotoxic material and/or machinery that supports proteostasis across cells and how, upon ageing, the ability to sustain a robust proteostasis networks is lost.
In our lab we use the retina as a privileged model to study transcellular proteostasis. The retina is a highly specialized tissue with a wide variety of postmitotic cells, including the Retina Pigmented Epithelium (RPE). RPE cells provide trophic support to photoreceptors, while ensuring phagocytosis of the shedding photoreceptor outer segments. We believe that loss of proteostasis by ageing RPE cells plays a critical role in degeneration of the photoreceptors, leading to loss of vision in ageing individuals such as in Age-related Macular Degeneration (AMD), which is still the leading cause of vision loss in the elderly in developed countries. In this context we are investigating how transcellular proteostasis contributes to RPE and overall retinal fitness, while trying to understand how we might improve proteostasis during ageing to prevent retinal degeneration and AMD.
Figure 1
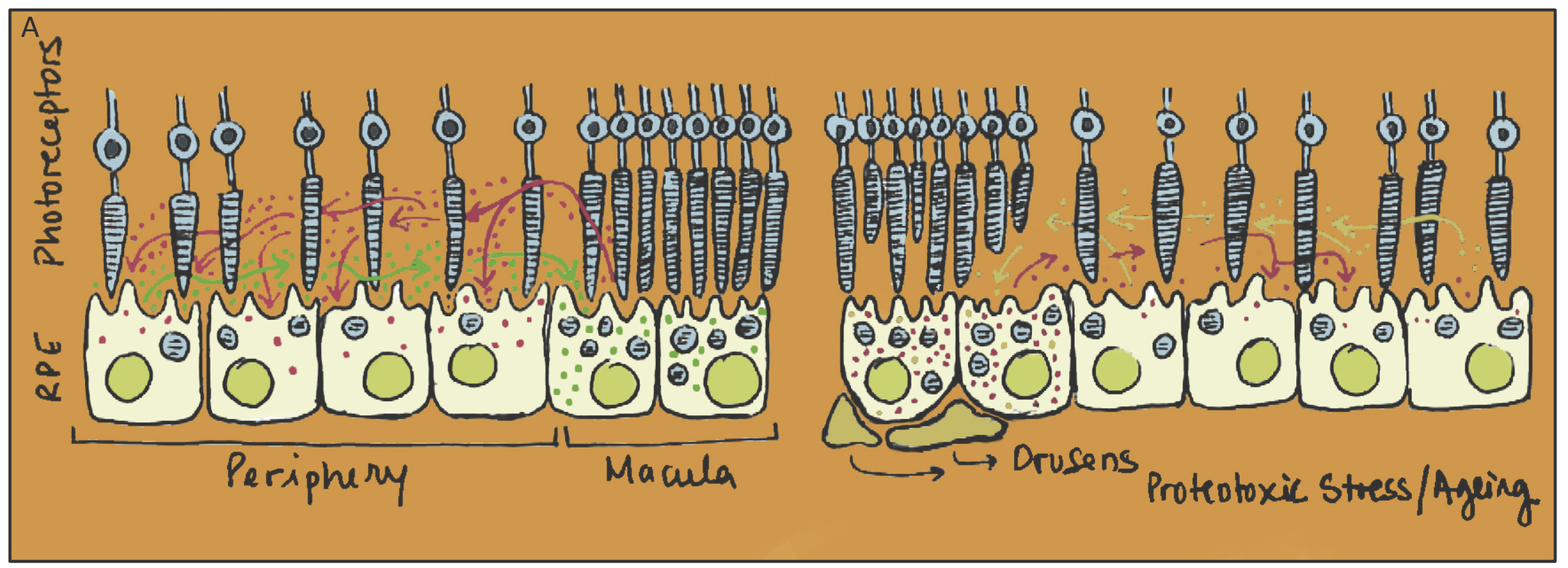
Legend
The RPE cells in the retina are subject to an increased burden in the macula (as the concentration of photoreceptors is higher). We propose that RPE cells use exosomes to share and redistribute both proteotoxic material and chaperone machinery across the RPE monolayer so that cells with intact proteostasis networks assist overburden RPE in the macula to cope with proteotoxic material, either by incorporating exosomes containing proteotoxic material (red arrows) or by releasing exosomes containing machinery that supports proteostasis (green arrows)
Recently we have also been interested in the molecular mechanisms whereby cells load specific proteins into exosomes. There is ample evidence suggesting that the cargo repertoire of exosomes does not necessarily reflect the cytosolic contents of the originating cell, indicating the existence of some type of active sol