1. PtCRIN
An infrastructure dedicated to improve national clinical research by promoting a more efficient implementation of clinical trials and particularly international cooperation. Multinational clinical trials provide the evidence necessary to approve new drugs and medical devices but require complex technical competences. PtCRIN implemented a network of academic clinical trial units (CTUs) in Portugal to deal with these requirements and linked to European counterparts (ECRIN-ERIC).
Clinical experimental studies (trials) provide the highest level of evidence to support the approval of innovative health technologies (drugs, cell therapies, devices, food, behaviour). Experimental studies require 2 types of infrastructures: clinical sites (CRC) that manage the patients and implement the studies, and clinical trial units (CTUs or CROs) that manage the whole study from the design, funding, regulatory approvals, implementation, monitoring, publication, etc. PtCRIN created at the national level the concept of CTUs that provide services at not-for-profit rates to public sponsors and SMEs.
PtCRIN is a consortium of 10 national R&D institutions that host CTUs providing general services for clinical studies or equivalent units limited to the provision of specific services (statistics, informatics). The consortium has been already working in close articulation with an additional 11 affiliated health care units but is open to all.
PtCRIN's CTUs follow the standards/certification of the counterpart European infrastructure (ECRIN-ERIC) promoting the capture of international funds, fostering international cooperation and making Europe a single area for clinical research.
PtCRIN privileges investigator independent clinical trials (IICTs) across all disease. These trials provide an unbiased assessment of preventive, diagnostic and therapeutic interventions that might not otherwise be investigated and are relevant to policymakers and to real-world clinical practice.
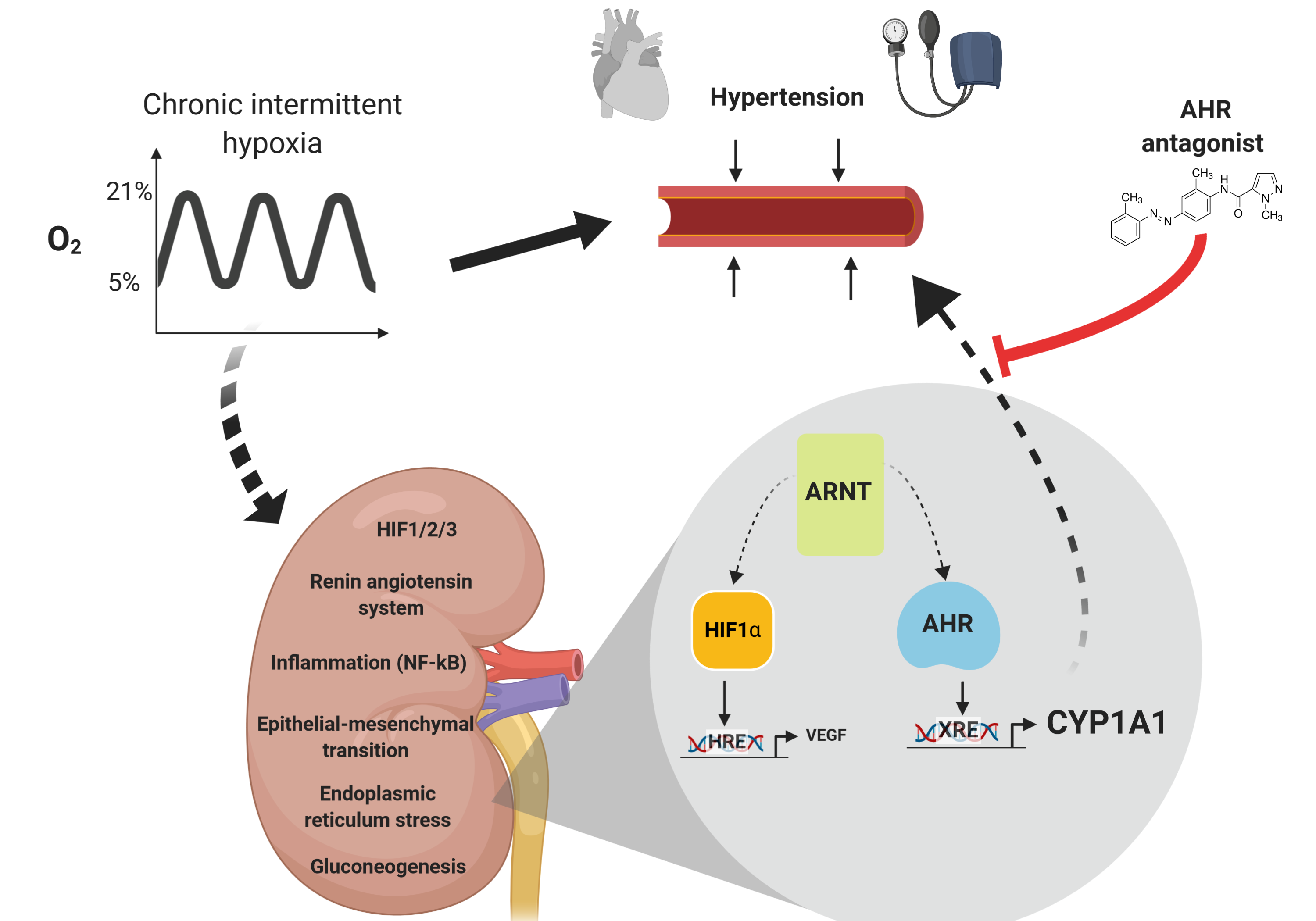
2. AhR-CYP1A:1 linking environmental exposure and sleep apnea to improve arterial hypertension management (PTDC/MED-TOX/30418/2017)