Bárbara Mendes, Diana Sousa and João Conniot are all first authors of a brand new review article in Trends in Cancer, supervised by João Conde at the Cancer Nanomedicine Lab, part of the ToxOmics research unit.
João Conde, Bárbara Mendes, João Conniot and Diana Sousa
They describe the latest developments in the use of nanoparticle-based technology for the treatment of cancer, particularly in the need to overcome the instrinsic variations in the tumor microenvironment. Their article is titled "Nanomedicine-based strategies to target and modulate the tumor microenvironment" and is available here.
What led you to prepare and publish this review?
With this review we intend discuss the groundbreaking advances in the field of nanotechnology with relevance to cancer diagnosis and therapy, as well as exploring potential targets for novel anticancer nanomedicines.
Why is this important?
Cancer nanomedicine strategies have increased in the last decades. However, translating these nanotechnology-based approaches into clinically reliable therapies has been challenging. This is not only due the tumor heterogeinity but also because tumor progression is fostered by the intrinsic tumor microenvironment (TME), a complex and intricated network that creates a niche for tumor cell progression. Nanomedicine approaches, including engineered nanoparticles (NP) intend to strategically target relevant players of this microenvironment, to dismantle this structure and tackle cancer.
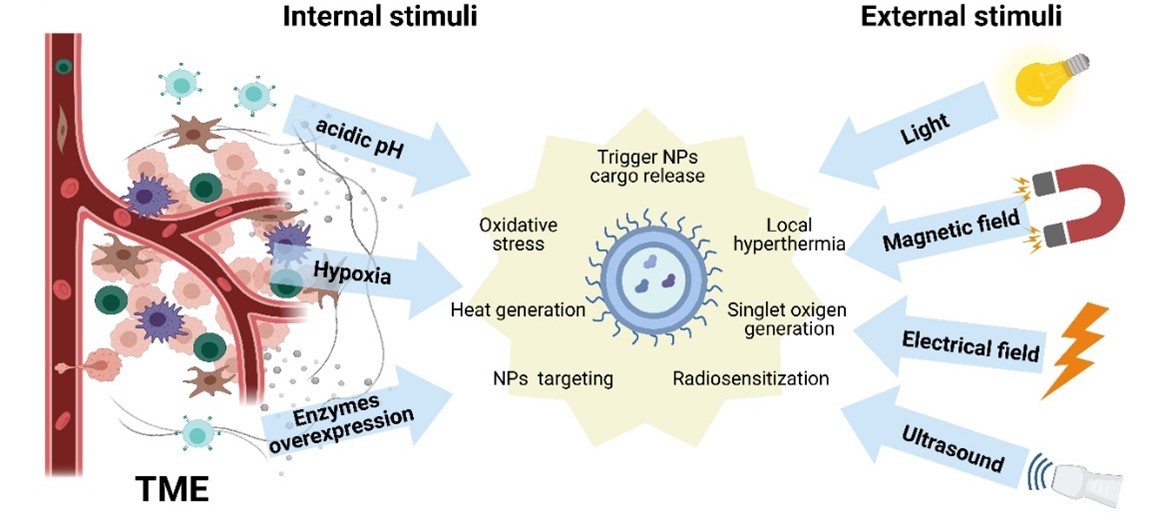
Schematic illustration of the internal and external stimuli for triggering therapeutic effects of nanotechnology-based systems. Created with BioRender.com
Can you use an analogy to help us understand this field?
This idea of Nanomedicine started more than 60 years ago, when in December of 1959, Richard Feynman (an American theoretical physicist) gave a lecture called “There's Plenty of Room at the Bottom” at an annual meeting of the American Physical Society at Caltech. In this well-known talk, Feynman placed the theoretical foundations for the field now called NanoMedicine when he could perceive the world where things could be miniaturized, or when huge volumes of intel could be programmed onto more and even more small devices, and when machines could be made significantly reduced and compact. Even though 1959 was the time when computers were the size of entire rooms, Feynman asked his audience: “I don't know how to do this on a small scale in a practical way, but I do know that computing machines are very large; they fill rooms. Why can't we make them very small, make them of little wires, little elements, and by little, I mean little?”.
In fact, the concept of miniature medical minions isn’t new. Richard Feynman at that time already suggested the possibility of “swallowing the doctor”. Feynman told us “it would be interesting in surgery if you could swallow the surgeon (small machine). (…) It goes into the heart and looks around. It finds out which valve is the faulty” and repairs it”. That inspired others to consider the possibility of manipulating individual atoms as a powerful tool for synthetic chemistry and synthetic biology. And this is the goal of NanoMedicine for cancer…differentially combat cancer using smart and targeted platforms that mediate highly selective therapies within the tumor microenvironment. The lack of standardized means to treat and profile the tumor microenvironment calls for a paradigm shift in the way we view and treat cancer.
What questions remain to be asked in this field that the group will pursue?
In our review article we summarized the biggest outstanding questions regarding this research, including:
- What barriers must be overcome for nanoengineered materials to reach their target in the tumor?
- What are the main challenges for the development of novel nanotechnology-based approaches in cancer?
- What are the advantages of nanomedicine for cancer therapy and diagnosis?
- What are the main strategies to improve cancer therapy and diagnosis through nanoengineered materials?
- What are the main obstacles to the clinical translation of cancer nanomedicines?
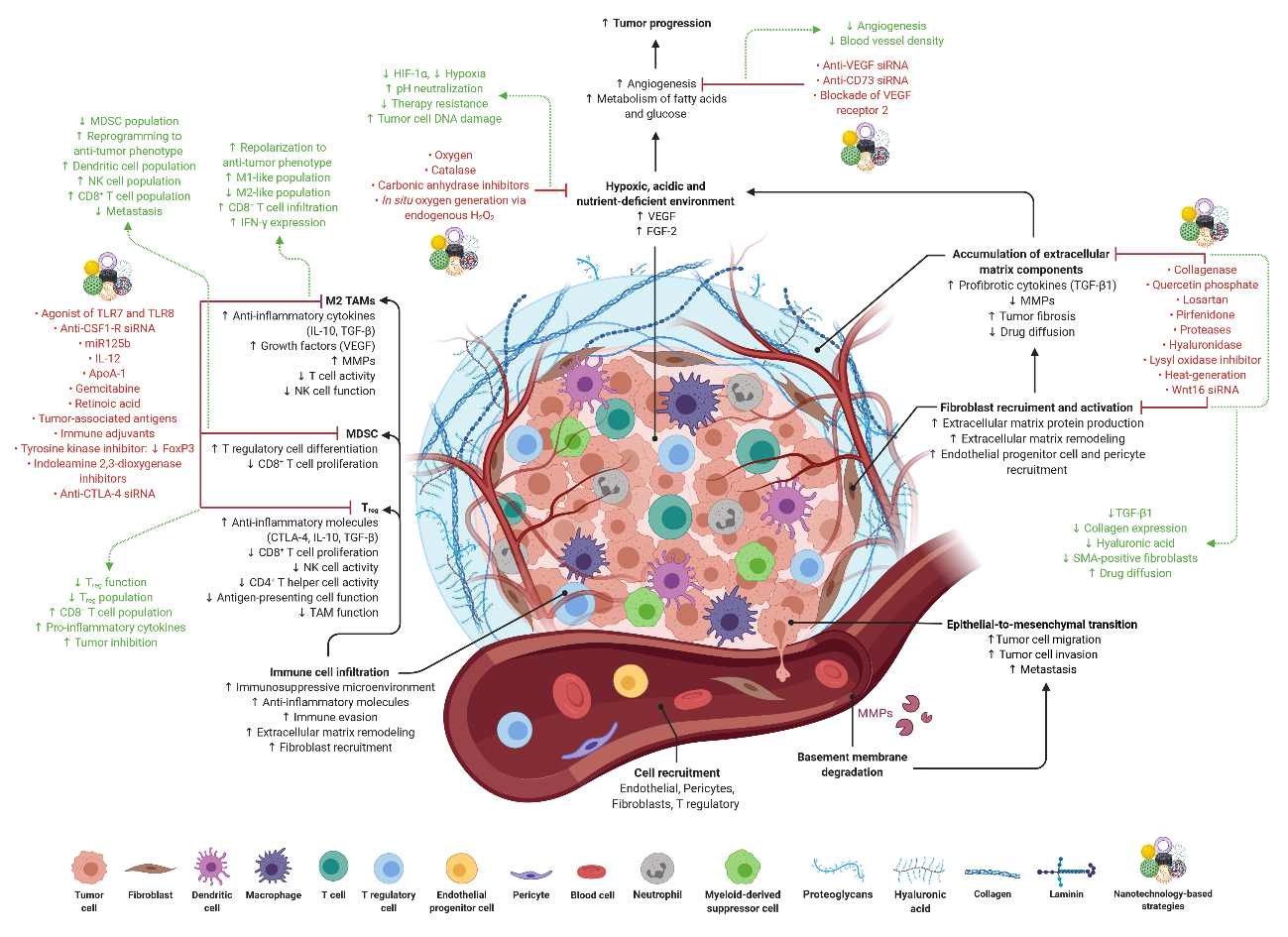
Schematic representation of the tumor microenvironment (TME), which is composed of stromal and immune cells and extracellular matrix components, among other, that are involved in metabolic, cellular and tissue remodeling. In the last years, Nanomedicine-based strategies have been developed to target and/or modulate TME.